Aim: To show that hydrogen gas is produced when a metal reacts with acid
Equipment: A test tube, A boiling tube, A Buncen burner, A wooden splint, A bottle of acid, A piece of metal, Safety glasses.
Hypothesis: I think that when we mix acid and metal, the metal with start to get eaten by the acid and it will become a gas and bubble up. and if i put a match to it it will make a pop sound.
Method:
1. Light your buncen burner
2. Add your sample metal to your test tube. add 2 mL of acid
3. Carefully invert the boiling tube above the test tube containing the metal and acid
4. Hold the test tubes together for a few minutes, allowing time for the inverted boiling tube to fill with gas.
5. When you think the tube if full. your lab partner should light a wooden splint.
6. Carefully and quickly tilt the boiling tube full of gas upwards and insert the burning splint into the mouth of the test tube
things to be careful about:
Caution
Wear safety glasses
Explosive
Corrosion
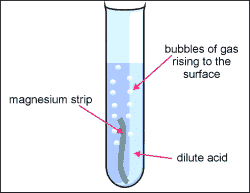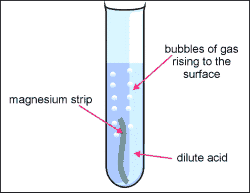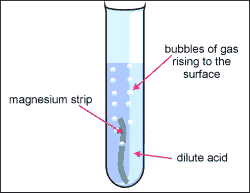
We saw the bubbling from the magnesium strip in the HCL And then we put the burning strip into the test tube and we heard a pop and this is because
The Hydrogen gas Combusted. My hypothesis was correct because when we mixed the metal with the acid it made the hydrogen gas.
Please leave feedback on this post so then i know what i can work on
Aidan this blog is looking great! You found a cool diagram to show the experiment and your observations are spot on. Add an intro to tell everyone what the purpose of the blog is.
ReplyDelete